Address
Seocho-daero, Seoul, South Korea
Work Hours
Monday to Friday: 10AM - 5PM KST
Weekend, Holiday: OFF
Address
Seocho-daero, Seoul, South Korea
Work Hours
Monday to Friday: 10AM - 5PM KST
Weekend, Holiday: OFF
Price range: $42 through $62
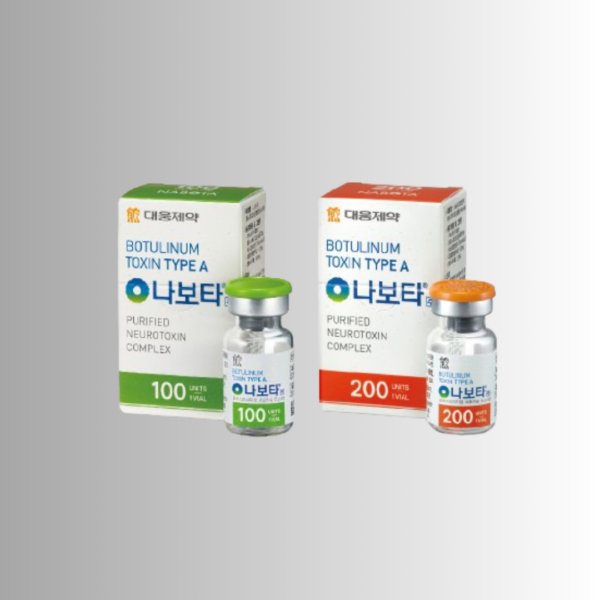
Nabota is a type A botulinum toxin that is used in the fight against severe wrinkles that are formed with uncontrolled muscle tone of the face and active facial expressions. Nabota is a safe, proven product released by Daewoong Pharmaceutical Co., Ltd, South Korea, which has more than 30 years of experience in biotechnology.
Component: Botulinum toxin type A
Package Unit: 1 Vial / BOX
Strengths:
Scope:
Duration:
The duration of the effect of 6 months and more.
Product composition:
Botulinum toxin type A
Packaging:
1 vial, 100 units per pack / weight: 30g
Manufacturer:
Daewoong Pharmaceutical Co. Ltd., South Korea
Strengths:
Scope:
Duration:
The duration of the effect of 6 months and more.
Product composition:
Botulinum toxin type A
Packaging:
1 vial, 200 units per pack / weight: 30g
Manufacturer: Daewoong Pharmaceutical Co. Ltd., South Korea
| Weight | 0.2 kg |
|---|---|
| Type | Nabota 100IU, Nabota 200IU |
Lucy Hernandez –
This is my 3rd time ordering Nabota 200 from LUNA EX. I love the product and fast delivery. Thank you as always.